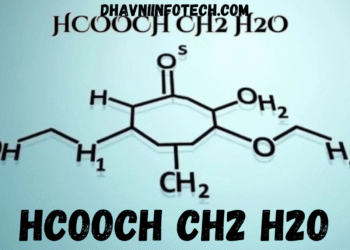
HCOOCH CH2 H2O is an organic compound that reflects a combination of formate, methylene, and water elements, symbolizing an interaction between an organic acid derivative and a hydrated group. The structure implies a compound that may form through esterification or condensation reactions involving formic acid and other simple organic molecules. This chemical blend represents a hybrid of reactivity and stability, making it an interesting subject for chemists studying reaction dynamics and organic transformations. It embodies the molecular bridge between formic acid and methanol derivatives, displaying a rich pattern of hydrogen bonding and weak intermolecular interactions that define its chemical essence.
Molecular Structure and Bonding Characteristics of HCOOCH CH2 H2O

The structure of HCOOCH CH2 H2O contains both polar and non-polar regions, giving it unique solubility and bonding features. The HCOO group contributes oxygen atoms capable of forming hydrogen bonds with the H2O molecule, which stabilizes the compound in aqueous media. The CH2 fragment introduces flexibility, enabling rotational freedom within the molecule. This dual property of hydrogen bonding and structural movement makes HCOOCH CH2 H2O reactive in controlled conditions, often taking part in hydrolysis or oxidation-reduction reactions. The presence of water molecules assists in maintaining equilibrium, preventing the compound from rapid decomposition or polymerization.
Physical Properties and Observable Behavior of HCOOCH CH2 H2O
Physically, HCOOCH CH2 H2O may appear as a colorless to pale liquid or crystalline solid depending on its hydration level and temperature. It exhibits a moderate boiling point, indicating intermolecular attraction influenced by hydrogen bonding. When exposed to air, the compound may gradually release a mild odor resembling formic acid or esters. **Its density, viscosity, and polarity are strongly governed by the amount
The Chemical Identity and Concept of HCOOCH CH2 H2O
HCOOCH CH2 H2O is a unique organic compound combining the structural features of formate, methylene, and water-based components, creating a balanced system between organic acid and hydrated molecules. It is often studied in organic chemistry as a representative compound illustrating esterification and hydrolysis reactions in equilibrium. This molecular combination exhibits distinct chemical characteristics because of the dual presence of oxygen and hydrogen atoms, which influence its reactivity, polarity, and stability in different chemical environments. It acts as a model compound for examining the link between formic acid derivatives and simple organic alcohols, making it valuable for academic and industrial chemistry studies.
Structural Framework and Molecular Interaction of HCOOCH CH2 H2O

The molecular structure of HCOOCH CH2 H2O demonstrates a delicate balance between ionic and covalent forces. The HCOO group (formate) provides polarity through carbon–oxygen double bonding, while CH2 contributes to the compound’s organic backbone, creating flexibility and mobility within its molecular design. Water (H2O) enhances intermolecular hydrogen bonding, stabilizing the molecule and influencing its reactivity in aqueous solutions. This combination of structural elements results in a compound capable of participating in multiple reaction pathways, including condensation and oxidation processes, essential in organic synthesis and industrial chemistry.
Physical and Chemical Properties of HCOOCH CH2 H2O
The compound HCOOCH CH2 H2O exists primarily in liquid or semi-hydrated crystalline forms, depending on temperature and surrounding conditions. It possesses moderate volatility, a characteristic ester-like odor, and solubility in polar solvents due to hydrogen bonding. Chemically, it can undergo slow decomposition when heated, forming simpler molecules like CO2, CO, and water vapor. Its chemical reactivity is influenced by pH levels, where acidic or basic environments can alter its structural configuration, leading to hydrolysis or further condensation reactions.
Reaction Mechanisms and Behavior of HCOOCH CH2 H2O
HCOOCH CH2 H2O takes part in various chemical reactions, notably esterification, hydrolysis, and oxidation. In the presence of acids or bases, it can hydrolyze to form formic acid and formaldehyde derivatives. When oxidized under controlled laboratory conditions, it yields carbon dioxide and other organic byproducts, showing its reactive nature. These reactions are important for understanding molecular transformation mechanisms and are widely used to illustrate organic reaction theories in advanced chemistry.
Industrial and Laboratory Applications of HCOOCH CH2 H2O

In industry and research, HCOOCH CH2 H2O has importance as a chemical intermediate, solvent, and model compound for studying formate chemistry. It plays a role in developing bio-based fuels, polymer precursors, and fragrance manufacturing. In laboratories, it is used in controlled experiments to observe hydrolysis rates, catalyst performance, and solvent interactions. The compound’s hybrid nature allows scientists to simulate complex organic behaviors in simpler systems, providing insight into real-world industrial reactions.
Environmental Interaction and Decomposition Patterns of HCOOCH CH2 H2O
When exposed to environmental conditions such as sunlight or air, HCOOCH CH2 H2O gradually breaks down into simpler carbon compounds. The decomposition is typically non-toxic, releasing carbon dioxide and water as end products. Its biodegradability makes it suitable for eco-friendly applications, particularly in green chemistry and sustainable solvent systems. The environmental fate of this compound highlights its compatibility with nature and minimal long-term residue effects, promoting safe use across industries.
Experimental Studies and Research Value of HCOOCH CH2 H2O
Scientists have conducted extensive experiments on HCOOCH CH2 H2O to explore its reaction kinetics, molecular stability, and thermodynamic properties. The results show its versatile nature as a research compound suitable for testing catalysts, energy storage reactions, and organic transformation patterns. It also serves as an educational example for chemistry students to understand hydration, ester formation, and intermolecular bonding. In recent studies, HCOOCH CH2 H2O has been evaluated for its potential use in sustainable energy models and bioreactor systems.
Safety and Handling Considerations of HCOOCH CH2 H2O
Though relatively mild, HCOOCH CH2 H2O should be handled with care, avoiding prolonged exposure to open air or heat sources. In laboratory use, it requires appropriate storage in sealed containers to prevent unwanted hydrolysis or evaporation. Safety measures include using gloves, goggles, and proper ventilation during experiments. Its low toxicity profile allows safe disposal after neutralization, but large-scale handling still requires adherence to chemical safety protocols to maintain laboratory and environmental integrity.
Importance of HCOOCH CH2 H2O in Modern Chemistry
The significance of HCOOCH CH2 H2O extends beyond its structural form—it represents a bridge between simple and complex organic systems. Chemists utilize it to explore reaction mechanisms, molecular modeling, and the role of water in stabilizing reactive organic compounds. In educational contexts, it helps explain core organic principles such as ester formation, hydrogen bonding, and solvent effects. Its balanced reactivity makes it an ideal compound for experiments demonstrating the foundation of organic chemistry and applied industrial processes.
Conclusion
In conclusion, HCOOCH CH2 H2O stands as a remarkable compound illustrating the harmony between organic molecules and water’s stabilizing power. Its role in hydrolysis, esterification, and oxidation showcases its versatility and importance across laboratory, educational, and industrial platforms. Its environmentally compatible nature and scientific utility make it a valuable part of modern chemical research.
FAQs
Q1: What is HCOOCH CH2 H2O used for?
It is used in research, solvent systems, and chemical synthesis.
Q2: Is HCOOCH CH2 H2O stable?
Yes, it is moderately stable under normal temperature but decomposes on heating.
Q3: Can HCOOCH CH2 H2O be used in green chemistry?
Yes, its biodegradability and low toxicity make it suitable for eco-friendly applications.
Q4: What reactions does HCOOCH CH2 H2O undergo?
It primarily undergoes hydrolysis, oxidation, and esterification reactions.
Q5: Why is HCOOCH CH2 H2O important in organic chemistry?
Because it demonstrates the relationship between formate compounds, water, and reaction dynamics.
Read More: Ingredients in Wullkozvelex – Understanding What Makes It Work
























Alright folks, give a look at taiking88! Solid platform, pretty good performance. The games look good, and registration was easy. Give them a shot taiking88
Good post. I leaarn something new aand challenging on sites I stumbleupon on a dailly basis.
It will always be exciting to read through articles from other writers and
practice something from other websites. http://boyarka-inform.com/
центр косметологии и хирургии клиника косметологии москва
Do you love gambling? https://cryptodepositcasinos.com allow you to play online using Bitcoin and altcoins. Enjoy fast deposits, instant payouts, privacy, slots, and live dealer games on reliable, crypto-friendly platforms.
Авторский блог https://blogger-tolstoy.ru о продвижении в Телеграм. Свежие гайды, проверенные стратегии и полезные советы по раскрутке каналов, чатов и ботов. Подробно о том, как увеличить аудиторию, повысить вовлеченность и эффективно монетизировать проекты в мессенджере Telegram.
институт косметологии клиника косметологии
Доброго времени суток.
Нашел любопытную статью.
Может кому пригодится.
Смотрите тут:
blacksprut зеркало
Вроде норм.
Погрузитесь в мир кино https://zonefilm.media с нашим онлайн-кинотеатром! Здесь каждый найдет фильмы для себя: от захватывающих блокбастеров и трогательных драм до мультфильмов для всей семьи. Удобный интерфейс, возможность смотреть онлайн на любом устройстве и постоянно обновляемая библиотека! Присоединяйтесь и наслаждайтесь!
Нужен сувенир или подарок? сувенирная продукция с нанесением для компаний и мероприятий. Бизнес-сувениры, подарочные наборы и рекламная продукция с персонализацией и доставкой.
Лучшие подарки и сувениры корпоративные подарки с логотипом на новый год нанесение логотипа, подарочные наборы, промо-продукция и деловые аксессуары для мероприятий и компаний.
Всем привет!
Нашел интересную тему.
Решил поделиться.
Вот ссылка:
блэкспрут рабочая ссылка
Вроде норм.
Нужна бытовая химия? профессиональная бытовая химия моющие и чистящие средства, порошки и гели. Удобный заказ онлайн, акции и доставка по городу и регионам.
Бытовая химия с доставкой магазин бытовой химии средства для уборки, стирки и ухода за домом. Широкий ассортимент, доступные цены и удобная оплата.
UEFA Champions League http://sampiyonlar-ligi.com.az/ matches, results, and live scores. See the schedule, standings, and draw for Europe’s premier club competition.
Football online http://soccerstand.com.az/ live match results and accurate online scores. Tournaments, schedules, and up-to-date team statistics.
Free online games http://1001.com.az/ for your phone and computer. Easy navigation, quick start, and a variety of genres with no downloads required.
Turkish Super League https://super-lig.com.az/ standings, match results, and live online scores. Game schedule and up-to-date team statistics.
Авто портал https://avto-limo.zt.ua с новостями автопрома, обзорами новых моделей, тест-драйвами и аналитикой рынка. Актуальная информация о ценах, комплектациях и технологиях для водителей и автолюбителей.
Автомобильный портал https://addinfo.com.ua свежие новости, сравнения моделей, характеристики, рейтинги и экспертные обзоры. Все о легковых авто, кроссоверах и электромобилях в одном месте.
Новости авто https://billiard-sport.com.ua тест-драйвы, обзоры и подробные характеристики автомобилей. Авто портал с аналитикой рынка, изменениями цен и новинками мировых брендов.
Современный авто https://comparecarinsurancerfgj.org портал: статьи о выборе автомобиля, сравнительные обзоры, советы по обслуживанию и ремонту. Информация для покупателей и владельцев авто.
Все об авто https://xiwet.com в одном портале: новости, тест-драйвы, рейтинги, комплектации и цены. Полезные статьи о выборе, обслуживании и современных технологиях.
Авто портал https://shpik.info с обзорами, сравнением брендов, характеристиками и аналитикой цен. Актуальные материалы для покупателей и автолюбителей.
Современный авто https://comparecarinsurancerfgj.org портал: статьи о выборе автомобиля, сравнительные обзоры, советы по обслуживанию и ремонту. Информация для покупателей и владельцев авто.
Автомобильный портал https://ecotech-energy.com с каталогом моделей, отзывами владельцев и тестами на дороге. Узнайте о новых технологиях, расходе топлива и особенностях комплектаций.
Автомобильный портал https://clothes-outletstore.com о новинках из Европы, Китая, Японии и Кореи. Тест-драйвы, изменения цен, аналитика рынка и подробные характеристики моделей.
Все об автомобилях https://fundacionlogros.org новости автопрома, обзоры новинок, аналитика рынка и советы по покупке. Удобная навигация и полезные материалы для автолюбителей.
Авто портал https://gormost.info о легковых авто, внедорожниках и электромобилях. Тест-драйвы, сравнения комплектаций, изменения цен и главные события отрасли.
Новости автомобильного https://impactspreadsms.com мира, обзоры моделей, краш-тесты и рейтинги надежности. Портал для тех, кто выбирает авто или следит за трендами рынка.
Автомобильный портал https://microbus.net.ua с экспертными статьями, сравнением авто и подробными характеристиками. Помогаем выбрать машину и разобраться в комплектациях.
Авто портал https://quebradadelospozos.com свежие новости, аналитика продаж, тест-драйвы и мнения экспертов. Обзоры бензиновых, гибридных и электрических моделей.
Портал про автомобили https://rusigra.org новинки автосалонов, обзоры, цены, сравнение моделей и полезные советы по эксплуатации и обслуживанию.
Женский портал https://ruforums.net о красоте, здоровье, отношениях и саморазвитии. Актуальные тренды, советы экспертов, психология и стиль жизни современной женщины.
Женский сайт https://saralelakarat.com с материалами о моде, уходе за собой, фитнесе и внутреннем балансе. Полезные статьи, обзоры и вдохновение каждый день.
Сайт для женщин https://chernogolovka.net о карьере, финансах и личностном росте. Практичные рекомендации, мотивация и поддержка для достижения целей.
Женский портал https://fancywoman.kyiv.ua о психологии отношений и гармонии в паре. Разбор жизненных ситуаций, советы по коммуникации и уверенности в себе.
Женский сайт https://female.kyiv.ua о модных тенденциях, создании образа и индивидуальном стиле. Подборки, рекомендации и актуальные решения сезона.
Здарова народ!
Увидел любопытную инфу.
Советую глянуть.
Смотрите тут:
кракен даркнет
Пользуйтесь на здоровье.
Сайт для женщин https://femalebeauty.kyiv.ua о здоровье, самочувствии и активном образе жизни. Советы по поддержанию энергии и баланса в повседневной рутине.
Женский портал https://gracefulwoman.kyiv.ua о саморазвитии и мотивации. Практики для повышения уверенности, управления стрессом и раскрытия потенциала.
Женский сайт https://happylady.kyiv.ua с экспертными статьями о красоте, косметике и уходе. Разбор средств, трендов и профессиональных рекомендаций.
Сайт для женщин https://lidia.kr.ua о современных трендах лайфстайла, психологии и стиле жизни. Вдохновение и полезные материалы без лишней информации.
Женский портал https://madrasa.com.ua для активных и целеустремленных женщин. Мода, отношения, карьера и развитие в одном информационном пространстве.
Женский сайт https://maleportal.kyiv.ua о гармонии тела и разума. Фитнес, уход, психология и советы для уверенного образа жизни.
Сайт для женщин https://mirwoman.kyiv.ua о личной эффективности и балансе между работой и отдыхом. Практичные советы и вдохновляющие истории.
Женский портал https://miymalyuk.com.ua о красоте, уверенности и современных трендах. Полезные статьи для ежедневного вдохновения и роста.
Здарова народ!
Нашел годную инфу.
Решил поделиться.
Вот ссылка:
Mega онион
Пользуйтесь на здоровье.
Женский сайт https://oa.rv.ua о стиле, самооценке и эмоциональном благополучии. Поддержка и актуальные материалы для каждой женщины.
Женский портал https://onlystyle.com.ua о красоте, психологии и личных границах. Советы экспертов, актуальные тренды и поддержка для женщин, которые выбирают уверенность и развитие.
Женский сайт https://prettiness.kyiv.ua о самоценности, стиле и внутреннем балансе. Практичные рекомендации по уходу, отношениям и личностному росту.
Сайт для женщин https://prins.kiev.ua о современной жизни, карьере и гармонии. Актуальные материалы о мотивации, уверенности и достижении целей.
Женский портал https://reyesmusicandevents.com о моде, уходе и эмоциональном интеллекте. Экспертные статьи, тренды и вдохновение для ежедневного роста.
Женский сайт https://trendy.in.ua о трендах, вдохновении и личном выборе. Поддержка в вопросах карьеры, отношений и самореализации.
Женский сайт https://womanfashion.com.ua о здоровье, фитнесе и внутренней энергии. Полезные советы, психология и лайфстайл для активной жизни.
Сайт для женщин https://womanonline.kyiv.ua о развитии личности, финансах и независимости. Поддержка и реальные инструменты для уверенного будущего.
Женский сайт https://expertlaw.com.ua о современных отношениях, психологии и личном пространстве. Практичные материалы для осознанных решений.
Женский портал https://ww2planes.com.ua о стиле жизни, красоте и самореализации. Контент для тех, кто хочет быть в гармонии с собой и миром.
Сайт для женщин https://lady.kyiv.ua о красоте, развитии и осознанности. Современный взгляд на жизнь, стиль и внутренний баланс.
Онлайн журнал https://mcms-bags.com для женщин: тренды моды, уход за собой, любовь, материнство, рецепты, саморазвитие и женская психология. Читайте актуальные статьи и находите вдохновение каждый день.
Портал о недвижимости https://all2realt.com.ua рынок жилья, новостройки, аренда, ипотека и инвестиции. Обзоры цен, аналитика, проверка застройщиков, юридические нюансы и практичные советы для покупателей и продавцов.
Сайт для родителей https://babyrost.com.ua и детей о развитии, обучении, играх и семейных ценностях. Полезные советы, разбор сложных ситуаций, подготовка к школе и вдохновение для гармоничного воспитания.
Развивающий портал https://cgz.sumy.ua для детей и родителей — обучение через игру, развитие мышления, речи и творчества. Полезные задания, советы специалистов, материалы для дошкольников и школьников.
Клуб Молодих Мам https://mam.ck.ua пространство общения, поддержки и полезной информации. Беременность, роды, развитие ребенка, здоровье мамы и семейная жизнь — всё в одном месте.
Современный портал https://spkokna.com.ua для родителей и детей: воспитание, развитие, образование, досуг и безопасность. Актуальные статьи, советы специалистов и полезные материалы для всей семьи.
Лечение диабета https://diabet911.com современные методы контроля уровня сахара, питание, медикаментозная терапия, инсулин и профилактика осложнений. Полезная информация для пациентов и их близких.
Медицинский портал https://novamed.com.ua о здоровье: симптомы и диагностика заболеваний, методы лечения, профилактика и рекомендации врачей. Достоверная информация для пациентов и их близких.
Медицинский сайт https://pravovakrayina.org.ua о здоровье человека: диагностика, лечение, профилактика, лекарства и образ жизни. Проверенные статьи и актуальные рекомендации специалистов.
Строительный портал https://kompanion.com.ua всё о строительстве и ремонте: проекты домов, материалы, технологии, сметы и советы специалистов. Практичные решения для частного и коммерческого строительства.
Портал о медицине https://una-unso.cv.ua и здоровье — симптомы, причины заболеваний, рекомендации по лечению и поддержанию организма. Простая и понятная информация для пациентов.
Строительный сайт https://mtbo.org.ua о проектах домов, фундаментах, кровле, утеплении и отделке. Советы мастеров, расчеты, инструкции и актуальные решения для качественного строительства.
У каталозі https://kitchen.lviv.ua доступні ліжечка для немовлят у Львові
У прайсі https://dahfasad.top подана ціна утеплення фасадів за м2
У сервісі https://www.remontlviv.top представлені бетонні роботи у Львові
Купить сантехнику онлайн https://danavanna.ru широкий ассортимент оборудования для дома и ремонта. Современный дизайн, выгодные цены, акции и профессиональная консультация.
Нужен кондиционер? сплит система купить хабаровск “ТопКлиматДВ” – это интернет-магазин климатического оборудования и сопутствующих услуг с поставкой в любой регион России. В нашем магазине вы найдёте продуманный отборный ассортимент современного климатического оборудования, высокое качество предоставляемых услуг, низкие цены, возможность срочной поставки и монтажа оборудования. На все позиции мы даём длинную гарантию. Наше кредо – основательность и надёжность!
interior design needed? https://aktis.design custom projects, 3D visualization, material selection, and construction supervision. We create stylish and functional spaces for comfortable living.
Buy or sell real estate? https://aktis.estate luxury and country real estate in prime locations. Detailed descriptions, photos, prices, and secure transaction assistance. We’ll find the perfect home for living or investing.
Do you want to relax? https://holidaygreece.eu rent a house or villa by the sea – comfortable accommodations, beautiful locations, and the unforgettable atmosphere of Greek resorts.
I recommend pin-up online casino for anyone who values privacy and security, as they use high-level encryption for all financial transactions. It feels like a safe environment for both casual and high-stakes players.
Медицинский сайт https://nogostop.ru об анатомии, патологиях и способах лечения. Симптомы, профилактика, современные препараты и рекомендации врачей в доступной форме.
Информационный портал https://diok.ru о событиях в мире, экономике, науке, автомобильной индустрии и обществе. Аналитика, обзоры и ключевые тенденции.
шумоизоляция арок авто https://shumoizolyaciya-arok-avto-77.ru
выездной шиномонтаж 24 https://vyezdnoj-shinomontazh-77.ru
Сайт о фермерстве https://webferma.com и садоводстве: посадка, удобрения, защита растений, теплицы и разведение животных. Полезные инструкции и современные агротехнологии.
Новости и обзоры https://mechfac.ru о мире технологий, экономики, крипторынка, культуры и шоу-бизнеса. Всё, что важно знать о современном обществе.
Все об автозаконах https://autotonkosti.ru и штрафах — правила дорожного движения, работа ГИБДД, страхование ОСАГО, постановка на учет и оформление сделки купли-продажи авто.
Онлайн новостной портал https://parnas42.ru с актуальными новостями, экспертными комментариями и главными событиями дня. Быстро, объективно и по существу.
Мировые новости https://m-stroganov.ru о технологиях и криптовалютах, здоровье и происшествиях, путешествиях и туризме. Свежие публикации и экспертные обзоры каждый день.
Ежедневный обзор: https://paradstars.com/catalog/219/
Хочешь продать недвижимость? квартиры у метро в Москве экспертная оценка, подготовка к продаже, юридическая проверка и сопровождение на всех этапах сделки.
джеттон геймс промокод
Нужен новый телефон? айфон спб по выгодной цене. Интернет?магазин i4you предлагает оригинальные устройства Apple с официальной гарантией производителя — от года и более. Интернет?магазин i4you: оригинальные устройства Apple с гарантией от года. Выбирайте лучшее!
Сервисный центр Miele в Москве предоставляет полный комплекс услуг по ремонту и техническому обслуживанию бытовой техники премиального уровня. Специалисты работают со стиральными и сушильными машинами, посудомоечными системами, духовыми шкафами, кофемашинами и холодильным оборудованием. Каждая заявка начинается с профессиональной диагностики, которая позволяет точно определить причину неисправности и подобрать оптимальное решение. Узнать подробнее можете по ссылке – ремонт техники миле
https://labirint-kids.com.ua/
Официальный сервисный центр Miele выполняет ремонт с использованием оригинальных комплектующих и специализированного оборудования. Это особенно важно для техники с электронными модулями управления и сенсорными панелями, где требуется точная настройка. Мастера регулярно проходят обучение и знают особенности разных модельных линеек бренда. Узнать подробнее можете по ссылке, https://mieleservis.ru/
https://adidas-store.ru/
Реклама на автомобиле стоимость https://oklejka-transporta.ru
https://itcent.com.ua/
https://goodtech.com.ua/
https://b-mobile.com.ua/
https://kotok.com.ua/
https://digital-world.com.ua/
https://poehali.com.ua/
Мы пишем о детях и воспитании с вниманием к деталям, рассказываем о новинках индустрии красоты и помогаем ориентироваться в мире моды. Наш журнал создан для вдохновения и поддержки. Переходите по ссылке и открывайте новые материалы https://universewomen.ru/
Проблемы с зубами? стоматологический центр профилактика, лечение, протезирование и эстетическая стоматология. Забота о здоровье зубов с применением передовых методик.
гражданство румынии недорого
https://mylikari.com.ua/
A professional house renovation company Moraira can transform an outdated property into a modern luxury villa. As a leading renovation company Moraira, we bring expert craftsmanship to modernize your kitchen or living areas, significantly increasing your home’s market value.
Охраны труда для бизнеса учебно методический центр аудит системы безопасности, обучение персонала, разработка локальных актов и внедрение стандартов. Помогаем минимизировать риски и избежать штрафов.
Нужен фулфилмент? https://mp-full.ru — хранение, сборка заказов, возвраты и учет остатков. Работаем по стандартам площадок и соблюдаем сроки поставок.
https://mama-choli.com.ua/
Запчасти для сельхозтехники https://selkhozdom.ru и спецтехники МТЗ, МАЗ, Амкодор — оригинальные и аналоговые детали в наличии. Двигатели, трансмиссия, гидравлика, ходовая часть с быстрой доставкой и гарантией качества.
https://ostrovturista.com.ua/
https://architector.com.ua/
https://agrotis.com.ua/
Орби казино https://orby-casino.com/ онлайн-платформа с широким выбором слотов, настольных игр и бонусных предложений. Узнайте об акциях, турнирах и возможностях для комфортного игрового досуга.
Онлайн казино Орби http://orby-casino.net/ большой выбор слотов, бонусы для новых и постоянных игроков, регулярные турниры с призами.
https://pohod.com.ua/
https://vy-doctor.com.ua/
Hey I am so excited I found your weblog, I really found you by mistake, while I was researching on Bing for something else, Anyways I am here now and would just like to say cheers for a remarkable post and a all round entertaining blog (I also love the theme/design), I don’t have time to read through it all at the moment but I have saved it and also added in your RSS feeds, so when I have time I will be back to read much more, Please do keep up the awesome job.
byueuropaviagraonline
https://goldmaster.com.ua/
https://nailsforyou.com.ua/
Чудові бонусы казино — депозитні бонуси, бездепозитні бонуси та Турнір з призами. Обзори пропозицій і правила участі.
Найпопулярніша платформа найкраще онлайн казино – популярні слоти, бонуси та турніри з призами. Огляди гри та правила участі в акціях.
Грайте в найкращі слоти — популярні ігрові автомати, джекпоти та спеціальні пропозиції. Огляди гри та можливості для комфортного харчування.
скільки заробляє пожежник в україні
Квартиры в новостройках https://domik-vspb.ru от застройщика — студии, однокомнатные и семейные варианты. Сопровождение сделки и прозрачные условия покупки.
Найкращі ігри в казино – безліч ігрових автоматів, правил, бонусів покерів і. Огляди, новинки спеціальні
https://medart.in.ua/
https://infobanks.com.ua/
pravdahub.com.ua/skilky-zarobliaie-dsns-ofitsiyna-zarplata-i-bonusy-riatuvalnykiv/